C2h6 Cl2 C2h5cl Hcl This Reaction Is Best Described as
Fundamental Concepts in Organic Reaction Mechanism. We do as follows.

Solved Ethan E Is Chlorinated In A Continuous Reactor C2h6 Chegg Com
The organic reaction.

. C2H6 Cl2 HCl. None of the above. 2 addition involving an unsaturated hydrocarbon.
Answer Expert Verifiedquestionquestion mark. C2H6 Cl2 C2H5Cl HCl Some of the product monochloroethane is further chlorinated in an undesired side reaction. Which answer is best.
Please correct your reaction or click on one of the suggestions below. Equation C2H6Cl2C2H5Cl is an impossible reaction. What will be the major products when C2H4 undergoes an addition reaction with Cl2.
C2H5Cl Cl2 C2H4Cl2 HCl. Instructions and examples below may help to solve this problem. Given the equation.
A Although the wavelength of light and the frequency of light can. C2H6 Cl2 C2H5Cl HCl. C2H6 Cl2 --- C2H5Cl HCl This reaction is best described as 1 addition involving a saturated hydrocarbon 2 addition involving an unsaturated hydrocarbon 3 substitution involving a saturated hydrocarbon.
H-Cl 431 Delta H rxn Summation BE reactants - Summation BE products. Ethane is chlorinated in continuous reactor. C2H6 Cl2 --- C2H5Cl HCl This reaction is best described as 1 addition involving a saturated hydrocarbon.
C2H6 Cl2 -- C2H5Cl HCl This reaction is best described as 1. C 6 H 5 COOH O 2 CO 2 H 2 O. The balanced chemical reaction is.
Examples of complete chemical equations to balance. 3 substitution involving a saturated hydrocarbon. K 4 Fe CN 6 H 2 SO 4 H 2 O K 2 SO 4 FeSO 4 NH 4 2 SO 4 CO.
This reaction is best classified as Adecreases Bincreases Cremains the same 3As an addition reaction occurs the number of electrons shared between carbon atoms ACH3COOH CH3OH CH3COOCH3 H 2O BC2H6 Cl2 C2H5Cl HCl CC3H6 H2 C3H8 DC6H12O6 2 C2H5OH 2 CO2 4Which is an example of an addition reaction. Some of the product monochloroethane is further chlorinated in an undesired side reaction. According to the given equation the best classified is addition.
Bond energies in kJmol. For a given reaction. Up to 24 cash back Given the equation.
Up to 24 cash back Given the equation. Correct answer to the question. 89 75 83 94.
Fe Cl 2 FeCl 3. Assume that the percentage conversion of the limiting reactant is 60 and the feed composition in. C2H6 Cl2 C2H4Cl2 HCl.
C2H6 Cl2 C2H5Cl HCl. C2H6 Cl2 --- C2H5Cl HCl This reaction is best described as 1 addition involving a saturated hydrocarbon 2 addition involving an unsaturated hydrocarbon 3 substitution involving a saturated hydrocarbon. Up to 24 cash back Given the equation.
The limiting reagent row will be highlighted in pink. What will be the major products when C2H2 undergoes an addition reaction with Cl2. The addition reaction means that two or more molecules combine together to form a large one usually in organic reaction.
C2H6 Cl2 C2H5Cl HCl. C2H5Cl Cl2 C2H4Cl2 HCl The reactor is designed to yield a 15 conversion of ethane and a selectivity of 14 mole C2H5Cl mole C2H4Cl2 with a negligible amount of chlorine in the product gas. KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2.
A What is the mole percent of HCl in the product. Substitution involving a saturated hydrocarbon 4. 2 on a question Given the equation.
Organic Chemistry - Some Basic Principles and Techniques. C2H4 undergoes an addition reaction with Cl2. C2H6 Cl2 C2H5Cl HCl This reaction is best described as 1 addition involving a saturated hydrocarbon 2 addition involving an unsaturated hydrocarbon 3 substitution involving a saturated hydrocarbon 4 substitution involving an unsaturated hydrocarbon Short Answer Car Chemistry.
4 substitution involving an unsaturated hydrocarbon. Substitution involving an unsaturated hydrocarbon. C2H6 Cl2 C2H5Cl HCl.
Addition involving an unsaturated hydrocarbon 3. C2H6 Cl2 C2H5Cl HCl This reaction is best described as 1 addition involving a saturated hydrocarbon 2 addition involving an unsaturated hydrocarbon 3 substitution involving a saturated hydrocarbon 4 substitution involving an. Question 16 3 pts Consider the following reaction C2H6g Cl2g C2H5Clg HClg if 125 g C2H6g reacts with 255 g Cl2g the percentage yield C2H5Cl actual yield 206 g C2H5Cl is.
Addition involving a saturated hydrocarbon 2. C2H6 Cl2 C2Cl6 HCl. C2H6 Cl2 C2H5Cl HCl This reaction is best described as Astarch and nylon Bstarch and cellulose Cprotein and nylon Dprotein and plastic 39Which polymers occur naturally.
Estimate the standard enthalpy change ΔH for the reaction below. Whats the Deal with Octane Ratings. We use the amounts given for the reactants as the starting point of the calculations to determine the limiting reactant.
Balance Chemical Equation - Online Balancer. Mole percent is 50 C2H6 40 Cl2 and 10 N2. Ethane is chlorinate in a continuous reactor.
Now consider the reactions C2H6 Cl2 C2H5Cl HCl C2H5Cl Cl2 C2H4Cl2 HCl Now consider the product as 100 moles and its composition in moles are Cl2 615 C2H6 231 C2H5Cl 115 C2H4Cl2 38 Where HCl is seperated. The reaction CH3CH3 Br2 CH3CH2Br HBr would best be described as. C2H6g Cl2g C2H5Clg HClg Data.
150 g C2H6 1 mol 3008 g 4987 mol 205 g Cl2 1 mol 709 g 2891 mol. Aaldehydes Besters Csynthetic polymers Dnatural polymers 40Cellulose protein and starch are classified as. C2H6 Cl2 HCl C2H5Cl is best described as.
The answer is 1 addition. A Suppose your principal objective is to maximize the selectivity of monochloroethane production relative to dichloroethane.
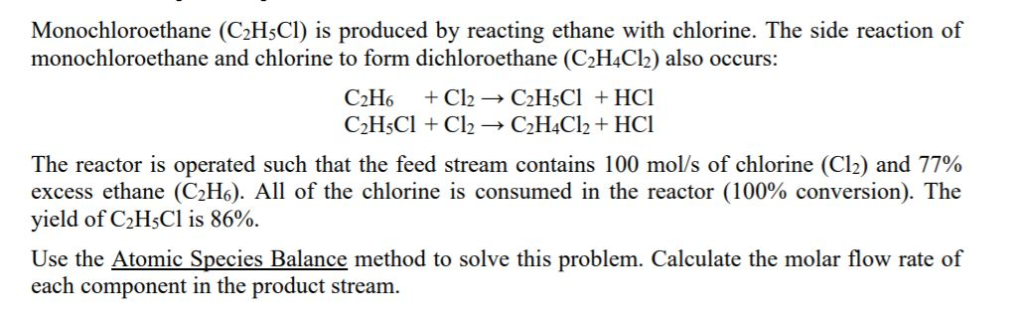
Solved Monochloroethane C2h5cl Is Produced By Reacting Chegg Com
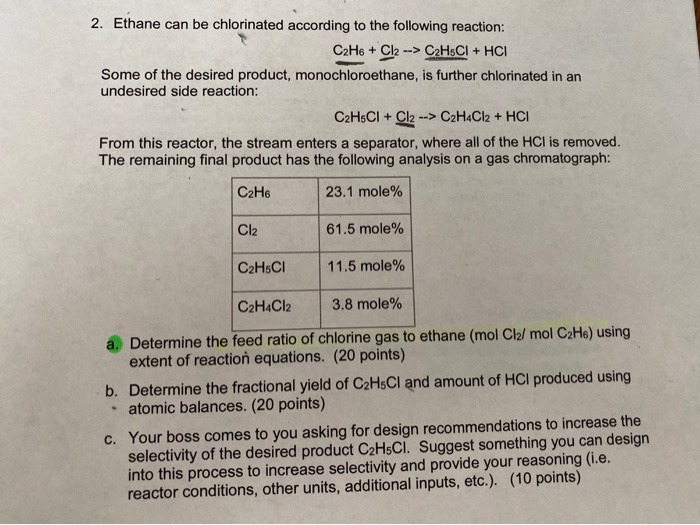
Solved 2 Ethane Can Be Chlorinated According To The Chegg Com

How To Balance C2h6 Cl2 C2cl6 Hcl Ethane Molecular Chlorine Youtube


Comments
Post a Comment